Introducing the Cordis 12 Holter ECG Monitor, the latest innovation by Recorders & Medicare Systems Pvt. Ltd., a renowned manufacturer and global supplier of top-quality Holter ECG Monitors in India. With our cutting-edge technology and commitment to excellence, we bring you a reliable and efficient solution for monitoring cardiac activity.
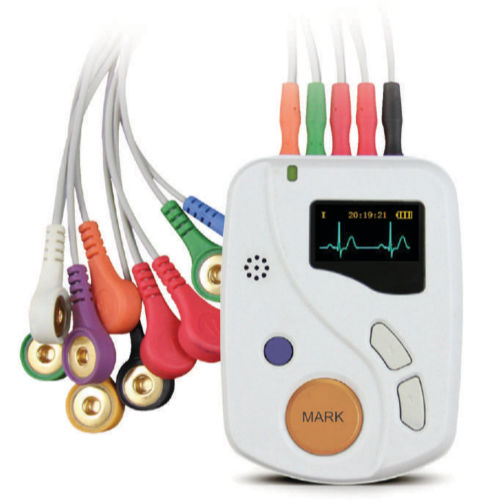
The Cordis 12 Holter ECG Monitor is a portable and wireless device that allows for long-term monitoring of the heart. It is designed to provide accurate and real-time data, making it an essential tool for healthcare professionals and patients alike. Whether you are a medical practitioner or an individual concerned about your heart health, this device is perfect for you.
Our Holter ECG Monitor is equipped with advanced features that ensure precise and comprehensive monitoring. It records and analyzes the electrical activity of the heart, providing valuable insights into any irregularities or abnormalities. With its 24-hour monitoring capability, it captures every heartbeat, allowing for a thorough analysis of cardiac function.
As a leading Holter monitor supplier in India, RMS understands the importance of reliability and accuracy in cardiac monitoring. That is why the Cordis 12 Holter ECG Monitor is built with state-of-the-art technology and adheres to the highest quality standards. It is a trusted companion for healthcare professionals in diagnosing and managing various cardiac conditions.
Not only does the Cordis 12 Holter ECG Monitor deliver exceptional performance, but it also offers convenience and ease of use. Its compact and lightweight design ensures comfort for patients during the monitoring period. The wireless feature allows for seamless data transfer, eliminating the hassle of tangled wires.
At RMS, we take pride in our commitment to customer satisfaction. As a global supplier, we cater to clients worldwide, including Zimbabwe. Whether you are in Harare, Bulawayo, Chitungwiza, Mutare, Epworth, Gweru, Kwekwe, or Kadoma, our Holter ECG Monitor is readily available to meet your needs.
Choose the Cordis 12 Holter ECG Monitor for accurate and reliable cardiac monitoring. Trust in the expertise of RMS, a leading manufacturer and distributor of Holter ECG Monitors. Take control of your heart health with our advanced technology and comprehensive solutions. Contact us today to learn more about our Holter ECG Monitor and how it can benefit you.

Reviews
There are no reviews yet.